Hydrogen (H
2) is a promising green fuel and a good way to store renewable energy, which can be easily released by combustion with molecular oxygen (O
2) producing just water (H
2O) as product of the reaction with zero-carbon footprint. Green Hydrogen can indeed be produced from water, using solar energy in a process mimicking photosynthesis. The sustainability of this upcoming technology however relies in the development of novel materials architectures that are together active, stable and based on Earth-abundant and cheap elements. Within the context of the “Make Our Planet Great Again” initiative, researchers at our Institute [
collaboration] have been one step closer with the preparation of a cost-effective photoelectrode for H
2 evolution made of p-type light-harvesting semiconductor covalently interfaced with earth-abundant molecular catalysts.
The strategy followed starts with the synthesis of a copper-iron oxide light harvesting semiconductor photocathode based only on Earth-abundant metals. This material was protected with amorphous TiO
2 (green circle in
Figure 1) deposited by Atom Layer Deposition (ALD). This thin film of TiO
2 (<10 nm) prevents the copper-iron oxide from corrosion while allowing the transfer of electrons towards a molecular cobaloxime catalyst (CoHEC) grafted at its surface (red circle in
Figure 1), following previous work from the team.
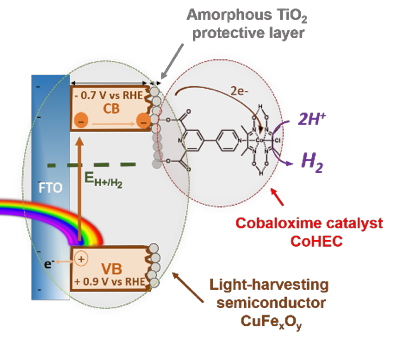
Figure 1: Scheme of electron pathway on CuFe
xO
y|TiO
2-CoHEC hybrid photocathode.
The resulting Earth-abundant element-based photoelectrodes evolve hydrogen from near-neutral aqueous solutions with quite positive onset photocurrent potentials (> 700 mV
vs RHE,
Figure 2) and their performance compare well with other recently reported systems. The collaboration with IRIG, institut Néel and EPFL researchers allowed for a detailed characterization of this photoelectrode architecture and a better understanding of its performances.
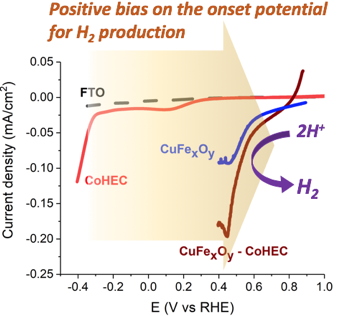
Figure 2: Linear Scan Voltammograms showing the positive bias on the onset potential when using the CuFe
xO
y-CoHEC.
Future work aims at enhancing the photocathode performance and to integrate it in a full photo-electrochemical system for self-sustained solar hydrogen evolution.
Collaboration: LCBM (Chemistry and Biology of Metals Laboratory, UGA, CNRS, CEA), Institut Néel (UGA,CNRS), PHELIQS (Quantum Photonics, Electronics and Engineering Laboratory, UGA, CNRS, CEA), SyMMES (Molecular Systems and nanoMaterials for Energy and Health, UGA, CNRS, CEA), LIMNO (Laboratory for Molecular Engineering of Optoelectronic Nanomaterials, EPFL, Switzerland).
A
p-type semiconductor has, due to intrinsic defects or added impurities (dopants) in small quantities, a slight electron deficit, or an excess of positively charged holes.
The Solar-Hybrid project is supported by the MOPGA postdoctoral program 2018 (Make our planet great again initiative) and DRF Impulsion 2018 program.