Among the new energy technologies, the use of hydrogen as an energy carrier represents a very attractive solution. However, an efficient hydrogen sector can only be develop if two key steps are mastered: firstly, the production of hydrogen in large quantities by electrolysis of water in devices called electrolyzers, and secondly, the use of hydrogen in fuel cells to provide electricity
via its oxidation. Currently, these processes require platinum as a catalyst (a catalyst is a substance that accelerates a chemical reaction). However, this metal is extremely rare (its abundance in the Earth's crust is approximately 5 ppm, similar to that of gold) and therefore very expensive. Therefore, getting rid of platinum and developing efficient catalysts containing only abundant and cheap elements is a major challenge for the future of the hydrogen economy.
In a previous study, IRIG researchers (in collaboration with IRAMIS and CEA-LITEN) combined nano-science and bio-inspired chemistry to develop a platinum-free material capable of catalyzing the production of hydrogen and its use in proton-exchange membrane fuel cells. They were able to immobilize a bioinspired nickel catalyst by covalent grafting onto carbon nanotubes acting as electrode materials.
To go further, they have developed, in a new
collaboration, another type of hydride material combining the same type of bio-inspired molecular catalyst with graphenic acid as a nano-structured electrode material. The bio-inspired catalyst used is directly inspired by the structure of the active site of hydrogenases (
Figure 1) from which it borrows both a nickel ion and pendant amines. It's between one of them and the nickel ion that the hydrogen molecule is activated (
Figure 2). The work carried out consisted in studying the interaction of graphenic acid carrying many carboxylic groups that can form salt bridges with guadinidium units installed on the bio-inspired catalyst (
Figure 2). Researchers also optimized the catalytic charge within the hybrid material to maximize the catalytic activity for hydrogen oxidation.
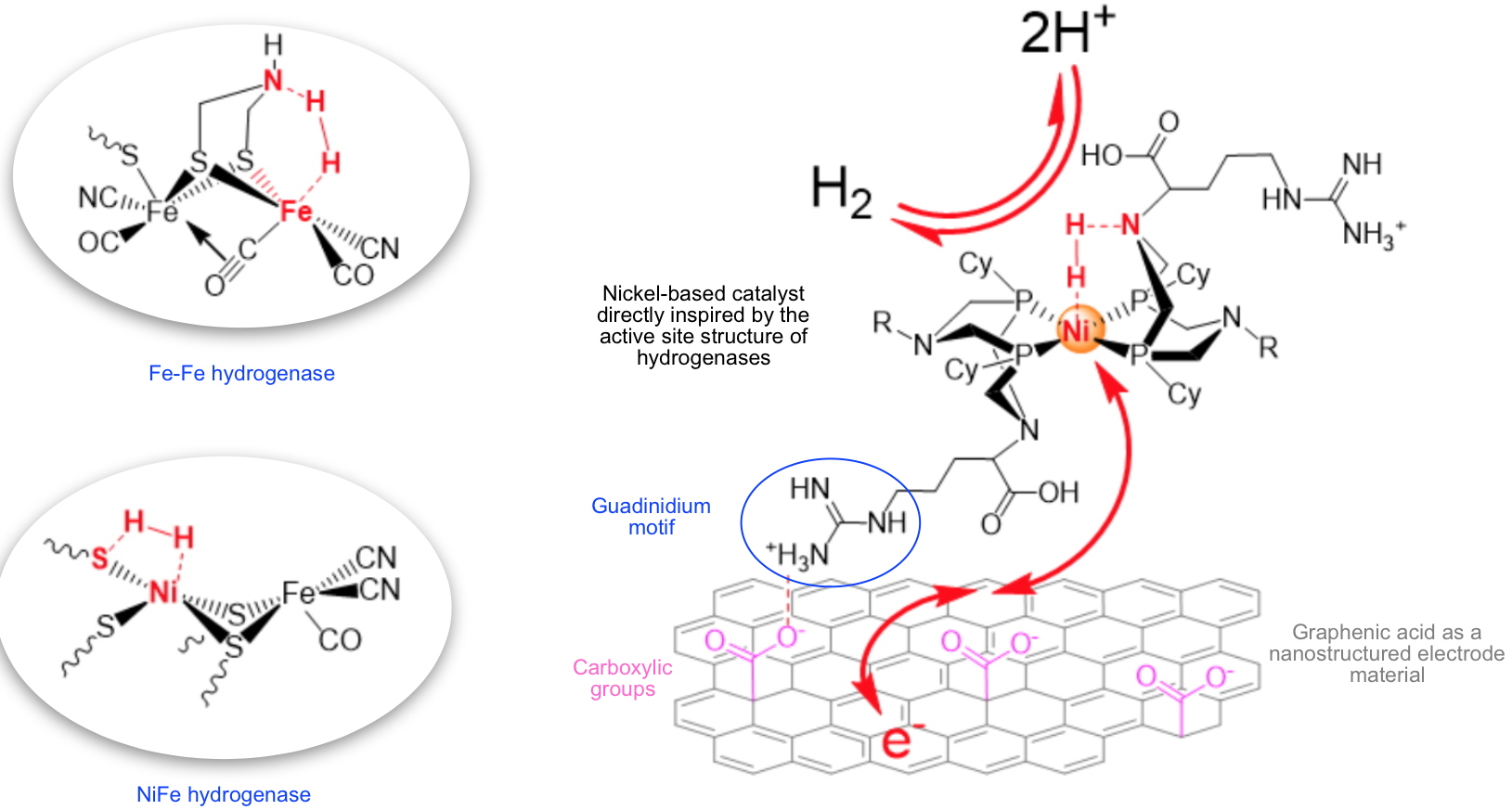
Figure 1 - Catalytic sites of FeFe and NiFe hydrogenases. | | Figure 2 - Schematic representation of the bio-inspired catalyst integrated into the hybrid material deposited on a gaseous diffusion layer. The bioinspired molecular catalyst is grafted to graphenic acid
via salt bridges which form between the carboxylic groups (in pink) of the graphenic acid and the guadinidium units (in blue) of arginine residues bear by the bioinspired catalyst. |
The new hydride material developed in this way offers record catalytic performance, superior to that previously achieved using carbon nanotube-based material. The study showed that this performance, although thirty times lower than that of platinum, was directly related to the catalytic charge and that it was therefore possible to continue increasing it by making thicker electrodes. However, it turned out to be difficult to manufacture such graphenic acid layers that are both mechanically stable and retain the same nanostructure, and therefore the same properties.
To meet the objectives of the
CRESCENDO and
BioPAC projects, researchers are now investigating new composite formulations including Nafion™ for integration into noble metal-free fuel cells.
Collaboration: Chemistry and Biology of Metals Laboratory (IRIG, CEA-Grenoble), Molecular Systems and nanoMaterials for Energy and Health laboratory (IRIG, CEA-Grenoble), Padova University (Italy) and Palacký University Olomouc (Czech Republic).
The
CRESCENDO project (GAN 779366) is supported by the Fuel Cells and Hydrogen 2 Joint Undertaking (FCH-JU,). The FCH-JU is funded by the European Commission's Horizon 2020 programme, Hydrogen Europe and Hydrogen Europe research.
The
BioPAC project is funded by the CEA Materials and Processes Transversal Programme of Competence (TPC).