Proteins are not rigid structures: they move, "breathe" and adapt to their environment in order to optimize their shape and activity. The existence of "breathing motions" by which proteins adjust their shape was highlighted in the 1970s by nuclear magnetic resonance (NMR) studies. Today, cutting-edge work in structural biology maps for the first time the structural details of such “breathing motions”. By combining NMR spectroscopy and X-ray crystallography, the research teams have mapped the structural changes, at atomic resolution, associated with rotations of aromatic rings located in the core of a protein. The study resolves a long-standing paradox in protein chemistry by showing how a void volume is generated around the aromatic amino acid to allow rotation of its side chain, while maintaining the structural fold of the protein.
This discovery sheds new light on how changes in the delicate balance of interactions that stabilize the hydrophobic core can lead to reorganizations of the protein structure. In addition, it offers a perspective on how novel biological functions can be acquired during evolution through modifications of the intricate network of interactions in the protein core. With the ability to "see" what is happening in the core of moving protein structures, we improve our understanding of how even small sequence alterations can be the basis of many diseases.
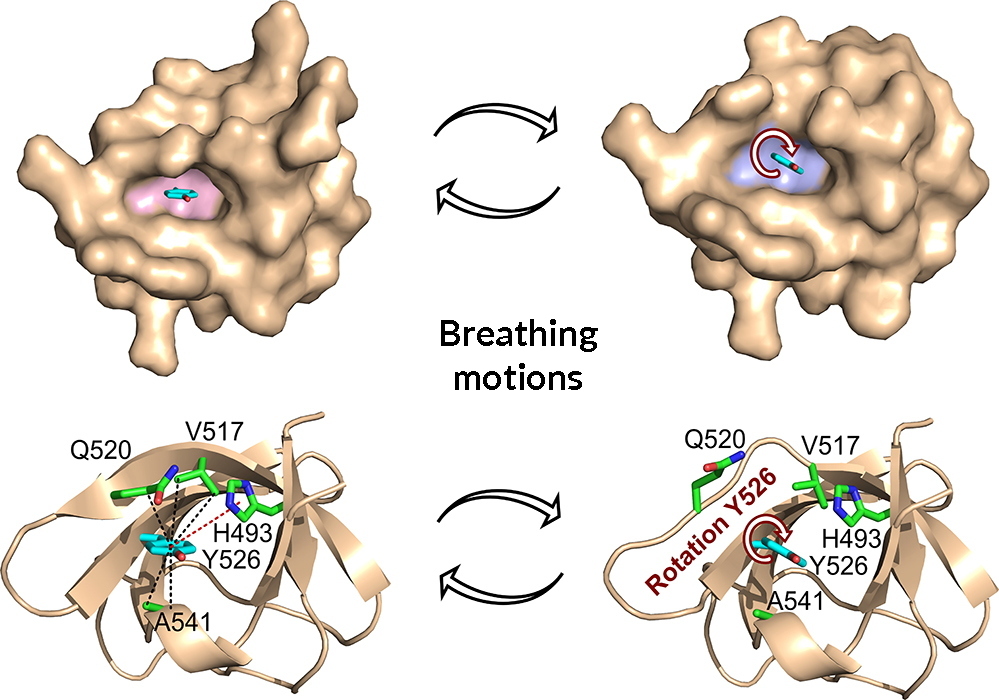
Visualization of breathing motions associated with rotations of the aromatic ring of tyrosine 526 (Y526) in the SH3 domain of the JIP1 protein. A void volume is created around the ring by distinct structural changes allowing ring rotations to occur.
© M.R. Jensen & A. Palencia